The energy industry is at an inflection point. With a finite supply of fossil fuels and growing demand for increased climate and environmental protection, the search is on for the next big thing in energy.
To many experts, hydrogen is it.
Hydrogen has promise as a carbon-free energy source with long-term storage potential. However, the main methods for producing it today rely heavily on coal and natural gas, making hydrogen a polluter and underscoring the need to produce more clean
hydrogen. Cost efficiency and other issues also hold back hydrogen’s potential as a major fuel source.
Today, hydrogen is used for a few specific purposes, including key industrial processes like producing ammonia for fertilizer. But government agencies and energy companies around the world are getting behind hydrogen, setting the stage for it to become more mainstream.
Texas is well-positioned to take advantage of this push toward hydrogen. Existing energy expertise, top-notch research institutions, an abundance of renewable energy and space for storage are all assets for the Lone Star State.
“If hydrogen’s going to work well anywhere, it should be in Texas. We have all the natural resources and existing infrastructure to make it work.”
-Michael Lewis, a senior engineering specialist in the Cockrell School of Engineering’s Center for Electromechanics
Lewis is one of more than 30 researchers at The University of Texas at Austin working on hydrogen projects. This group spans disciplines, including researchers from the Cockrell School of Engineering, Jackson School of Geosciences, College of Natural Sciences and UT’s Energy Institute. Together, they are looking at every aspect of hydrogen, from improving production, to efficiency, to deploying it in real-world processes.
Making Hydrogen Environmentally Friendly
One of hydrogen’s most appealing traits is the diversity of things it could power. Potential uses include fuel cells that could power vehicles, personal electronics and major industrial processes.
However, it has yet to reach that potential. Hydrogen use today is dominated primarily by industry, including oil refining, ammonia production, methanol production and steel production, according to a report from the International Energy Agency. Virtually all of this hydrogen is supplied using fossil fuels.
And that leads to a lot of pollution. According to IEA, hydrogen production is responsible for around 830 million tons of CO2 emissions per year, equivalent to the emissions generated by the United Kingdom and Indonesia combined.
If hydrogen is to become the next big thing in energy production, it needs to become more environmentally friendly. According to a recent study from the Jackson School, 98% of hydrogen is currently produced from natural gas and only 1% is made through electrolysis, a process that splits water into hydrogen and oxygen using electricity.
This means that hydrogen could be produced from solar, wind or other low-carbon electricity sources, but currently the technology is held back by cost and scaling issues.
New technologies for cost-effective, green methods of producing hydrogen would have a tremendous positive environmental impact on hydrogen production for current applications and enable even more uses,
said Edward Yu, professor in the Cockrell School and director of the Center for Dynamics and Control of Materials, a National Science Foundation Materials Research Science and Engineering Center. And the key barrier to widespread implementation is that it is still too expensive.
Hope is on the horizon in this regard. An IEA analysis found that the cost of producing hydrogen from renewable electricity could fall 30% by 2030 as a result of declining costs of renewables and the scaling up of hydrogen production.
Governments are lining up to support reducing the cost of producing clean hydrogen and making it a larger part of the energy mix. The European Union recently said last year that hydrogen will be a major part of its push to become carbon neutral by 2050. And U.S. energy leaders have pledged support for lowering the cost of clean hydrogen from $5 per kilogram to $1 per kilogram in a decade. Lawmakers have crafted legislation to encourage hydrogen use and development, with more incentives possibly on the way.
Making Hydrogen More Viable
This momentum comes as research breakthroughs across the world — and in labs on the UT campus — are bringing the potential of hydrogen closer to reality.
Last year, the U.S. Department of Energy’s H2@Scale program awarded a grant to researchers at UT Austin and a group of energy companies. The nearly $11 million project includes partnerships with Frontier Energy, Toyota, Shell and more.
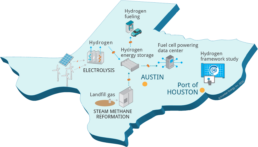
An illustration of Texas’ H2@Scale program.
As part of the project, UT Austin will host a first-of-its-kind integration of commercial hydrogen production, distribution, storage and use. The project partners will generate zero-carbon hydrogen at a new site via electrolysis with solar and wind power and reformation of renewable natural gas from a Texas landfill. It is the first time that both sources of renewable hydrogen will be used in the same project.
The hydrogen will power a stationary fuel cell to provide clean, reliable power for the Texas Advanced Computing Center and supply a hydrogen station with zero-emission fuel to fill a fleet of Toyota Mirai fuel cell electric vehicles.
We have seen a great amount of interest from industry, government and economic development groups in our project, and that shows a positive outlook for hydrogen in Texas and that industry is behind it,
Lewis said.
The team has selected a site and is working on building it out for a demonstration set for next year.
The other half of the H2@Scale project is a feasibility study for scaling up hydrogen production and use at the Port of Houston. The team will assess available resources, prospective hydrogen users, and delivery infrastructure, such as existing pipelines that supply hydrogen to refineries. The study will examine policies, regulations and economics to help industry develop a strategic action plan to present to policymakers to enable heavy-duty fuel cell transportation and energy systems.
Fuel cells, which convert the chemical energy of a feeder fuel such as hydrogen into electricity, have long been a point of interest for researchers at UT Austin. Years ago, students designed and built a fuel cell-powered UPS delivery truck. And the research has expanded since then.

The Center for Electromechanics’ fuel cell-powered UPS van.
In July, Guihua Yu, a professor of materials science in the Cockrell School’s Walker Department of Mechanical Engineering, published a paper detailing a new method to improve the oxygen reduction portion of the chemical reaction in fuel cells, in which O2 molecules are split to create water.
The researchers deployed a hydrogel anchoring strategy
that creates densely packed sets of iron atoms held in place by a hydrogel polymer. Finding the right formula for spacing these atoms created interactions that allowed them to morph into catalysts for oxygen reduction. Figuring out the density and locational dynamics of these iron atoms unlocks a level of efficiency in the fuel cell reaction never before realized.
Joan Brennecke, a professor in the McKetta Department of Chemical Engineering, is involved in a collaborative project with Los Alamos National Lab and Toyota. They are looking to improve the membranes in fuel cells and make it possible for the reaction to be performed at higher temperatures, which would make it faster and more efficient. In most practical applications like vehicles, you don’t want to have to think about cooling a fuel cell, as that would create a lot of challenges.
Brennecke has been working with Toyota for eight years, first on developing ionic liquids for batteries and later fuel cells. Transportation is an important part of the hydrogen equation, Brennecke said, because it is responsible for significant CO2 emissions. Transportation made up 29% of CO2 emissions worldwide in 2019, per the Environmental Protection Agency, the highest share of any major industry.
We’re getting to a point where mobile sources of CO2 need to be eliminated, and all those sources are in transportation,
Brennecke said. We need to run those on electricity, and we can do that by using hydrogen.
Brennecke isn’t the only researcher with Cockrell School ties looking at fuel cell membranes. A startup created to commercialize UT engineering research, called Celadyne, is also gaining momentum.
The company, founded by former graduate researcher Gary Ong and McKetta Department of Chemical Engineering Chair Delia Milliron, makes materials to improve hydrogen fuel cells and electrolyzers. Like Brennecke’s project, Celadyne aims to make membranes capable of withstanding higher temperatures.
Earlier this year, Celadyne got backing from Shell’s investment division, another sign that the larger energy industry is getting behind hydrogen.
Shell Ventures is actively looking at innovations in hydrogen. As a high-density energy carrier, hydrogen has an important role to play in delivering energy while lowering carbon emissions,
Phoebe Wang, ventures principal at Shell Ventures, said earlier this year. We are excited to work with Celadyne to further improve fuel cell technology and help accelerate the adoption of hydrogen solutions.
In addition to the fuel cell research, other engineers have taken aim at electrolysis, which has similar characteristics to the fuel cell reactions. In June, Edward Yu published a paper that also looked at the oxygen portion of electrolysis. The researchers found a low-cost way to use sunlight to efficiently split off oxygen molecules from water. The idea of using sunlight to split water molecules into hydrogen and oxygen has existed for decades, but the inability to find materials with the combination of properties needed for devices that can perform the key chemical reactions efficiently has kept it from becoming a mainstream method. Work in Yu’s lab offers the possibility of realizing cost-effective, highly scalable systems for direct generation of hydrogen and oxygen using solar power.
UT’s Bureau of Economic Geology is studying several aspects of hydrogen. The research institute within the Jackson School of Geosciences features an interdisciplinary team working on these projects, including from the Hildebrand Department of Petroleum and Geosystems Engineering Kamy Sepehrnoori, Mojdeh Delshad and Larry Lake. Their projects include:
Storage: Hydrogen carries only about a third of the energy of natural gas by unit volume, and so it requires more storage space. Importantly, hydrogen can be stored indefinitely, and geologic reservoirs offer the means to store large quantities of hydrogen. Currently, there are three subsurface hydrogen storage facilities with the capacity for industrial usage in the U.S., all of which are located in salt caverns along the Gulf Coast in Texas. However, more storage capacity will be required given demand expectations in a low-carbon economy.
The team wants to create an inventory of actual sites to evaluate for hydrogen storage feasibility and integrate it with an assessment of available gas pipeline networks. Knowing where storage and pipeline networks are feasible is a big step toward creating a roadmap for large-scale hydrogen use.
In-situ
combustion: This technique is used in extracting oil from the subsurface, and, under the right conditions, has been shown to produce hydrogen gas.
Using this method allows for the possibility of generating hydrogen underground, while keeping harmful byproducts like carbon dioxide buried. The researchers aim to model the process to understand the reactions created through combustion of hydrocarbons, identify reservoir types and fields useful for research, and develop and collaborate with materials researchers to develop membranes for underground hydrogen separation.
Hydrogen value chain: The researchers recognize a need to figure out where hydrogen fits into the larger energy picture. That includes an energy economic modeling system that looks at cost competitiveness and technology options to bring hydrogen to market.
Because hydrogen gas can be transported by pipelines, and large volumes can be stored in geological reservoirs indefinitely, it has the potential to serve current and emerging industrial uses as well as transportation and back-up power needs,
said Mark Shuster, deputy director of the bureau’s energy division. However, we are at a really early stage in understanding the reservoir geology and engineering of underground hydrogen-storage systems and being able to optimize development of these systems. We see subsurface research and development as a critical but, to date, somewhat overlooked part of the hydrogen game plan.
These projects, while all unique in scope, have similar goals: to improve the core processes involving hydrogen to make it more viable as a major energy source.
Texas, the Hydrogen Capitol?
With all this exciting research happening in Texas, the state is poised to become a leader in hydrogen innovation. It’s already an energy leader not just in the U.S., but worldwide.
And in Texas, there are already the bones of production and infrastructure for a hydrogen economy. Texas today produces more than 2 million tons of hydrogen annually, mostly as a byproduct of fossil fuels on the Gulf Coast. A huge network of hydrogen pipelines run through Texas.
In addition to infrastructure, Texas has the knowhow. The state’s expertise in oil and gas and renewables like wind and solar gives it a leg up on potential competitors. And the skillset for hydrogen fits well with the current oil and gas workforce, creating additional economic growth and job opportunities.
What’s needed next, researchers say, is increased policy support to incentivize the development of things like hydrogen fuel stations for vehicles and increased infrastructure. If this investment comes, Texas could develop a new identity as a hydrogen exporter to states across the country and nations around the world. But only if leaders act.
“There’s a story for hydrogen that Texas can get behind,” Lewis said. “But if Texas doesn’t do it, other states and countries will, and we’ll fall behind. Instead of being a leader, we’ll become a follower.”
by Nat Levy