In early 2020, the world’s spotlight shined on The University of Texas at Austin. Researchers at UT Austin became the first to decode and map the structure of the spike protein of the novel coronavirus, the part that allows SARS-CoV-2 to enter human cells. This breakthrough discovery, which came just weeks after the coronavirus genome was published online, went on to play an important role in several COVID-19 vaccines.
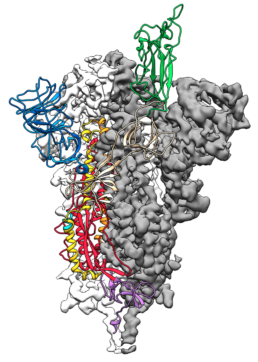
This is a 3D atomic scale map, or molecular structure, of the 2019-nCoV spike protein. The protein takes on two different shapes, called conformations—one before it infects a host cell, and another during infection. This structure represents the protein before it infects a cell, called the prefusion conformation.
What starts here changes the world, indeed.
It took just 66 days from the time the genome was published until the first vaccine trial began. Just 270 days after that, the Pfizer-BioNTech vaccine, which has ties to UT Austin, was approved for emergency use authorization by the U.S. Food and Drug Administration. Prior to this, the fastest a vaccine had gone from viral sampling to approval was four years, for mumps in the 1960s.
This blazingly fast effort, a galvanization of the scientific community rarely seen in modern times, benefitted from more than a decade of groundwork laid by engineers and scientists. Jason McLellan, professor of molecular biosciences in the College of Natural Sciences, started down this path searching for a vaccine to combat a respiratory virus called RSV and then applied what he learned to the first highly pathogenic coronaviruses that appeared, MERS and SARS-CoV.
These viruses are all made of genetic material coated in proteins. One of these, the spike protein, is essential for the virus to infect a cell. In the process of infection it changes shape. If the immune system encounters spike in the first shape, it makes potent antibodies that prevent infection, but not if the protein has taken on the second shape. McLellan and his colleagues mapped the structure of the RSV spike and figured out a way to freeze it in its first shape, leading to a new type of vaccine. Then they did it again for the MERS spike.

Jason S. McLellan, associate professor of molecular biosciences, left, and graduate student Daniel Wrapp, right, work in the McLellan Lab.
“As soon as we knew this was a coronavirus, we felt we had to jump at it to get this structure,” McLellan said last year. “We knew exactly what mutations to put into this, because we’ve already shown these mutations work for a bunch of other coronaviruses.”
This breakthrough focused global attention on UT Austin’s therapeutics capabilities. However, this is a burgeoning area of strength that UT researchers have been building for many years.
The business model for therapeutics is problematic because it has an expiration date on it,
said Jennifer Maynard, a professor in the Cockrell School of Engineering’s McKetta Department of Chemical Engineering and member of the vaccine research team. If we make a great vaccine, it eradicates the disease and then there is no longer a need for it.

Jennifer Maynard in her lab.
Maynard hopes that the speed of COVID vaccine approval will become a broader trend and that the field will move away from a model where it can take 10 to 15 years to get regulators to sign off on a therapeutic.
Hopefully this will change the way we approach infectious diseases,
Maynard said. Because this is really important; we still have a lot of other emerging diseases that are problematic around the world. We have malaria, tuberculosis; other infections that are becoming resistant to antibiotics.
Engineering Cures
The field of therapeutics/biologics includes several key treatments for patients: vaccines, antibodies, enzymes and peptides. UT experts in these areas have filed more than 100 U.S. patents in the last 10 years alone. Protein therapeutics spinoff companies from UT Austin have raised more than $750 million in the last five years to fund the extensive studies and clinical trials required for drug approval by the FDA.
This work has yielded multiple drug candidates that are being evaluated or are about to be evaluated in clinical trials and one that has been approved by the FDA. In 2016, the FDA approved a drug engineered by Maynard and George Georgiou, a professor in the McKetta Department and in the College of Natural Sciences’ Department of Molecular Biosciences who is leading the new Institute for Human Immunology and Protein Therapeutics, for the treatment and inhalation of anthrax.
The researchers, whose work began more than a decade ago, engineered high-affinity, sticky
antibody fragments that bind to the anthrax toxin, blocking its activity. They eventually licensed the technology to New Jersey-based company Elusys for further development.
Georgiou and Everett Stone, a research associate professor in the Department of Molecular Biosciences, invented three protein-based drugs for the treatment of debilitating genetic diseases that are now being evaluated in patients. In separate efforts, they have pioneered the development of therapeutic enzymes that serve to activate the immune system to fight cancer.
At UT Austin we have a rather unique culture of extensive collaborations between engineers and basic scientists that take a very holistic view of protein therapeutics development,
Georgiou said. We do not just come up with new concepts for therapeutics, as is the case in many other institutions, but we work hard to reduce them to practice and engineer the actual molecules that will be delivered to patients and also develop methods for their efficient production.
Maynard also developed a therapeutic treatment for pertussis (whooping cough). And she received the inaugural UT Austin Emerging Inventor of the Year
award in 2015 for her translational research. Stone also received this award in 2017.
These engineers and scientists are important players in the contribution to therapeutics at UT Austin, an effort that spans across disciplines. And that’s not the only aspect of treatments that Cockrell School researchers are studying closely.
UT Experts in Therapeutics
Getting Treatments Into the Body
Several of the COVID vaccines use mRNA – short for messenger RNA – to teach cells how to make copies of the spike protein that triggers an immune response in the body. That prepares the immune system to fight against the real thing.
There are very few RNA-based treatments that are authorized for use. But, they have tremendous potential, and how they are delivered into the body is a major area of interest for scientists and engineers around the globe, including Nicholas Peppas, a pioneering researcher in the field of drug delivery.
Peppas’ research combines modern molecular and cellular biology techniques with materials engineering to create next-generation solutions. His expertise includes biomaterials and bionanotechnology, and his work in the field of drug delivery has benefited patients around the world.

Nicholas Peppas and students in his lab.
Peppas is known for creating novel systems for insulin delivery to treat Type 1 diabetic patients. He and his fellow researchers have made breakthroughs in the delivery of treatments for a number of autoimmune diseases, including multiple sclerosis, Crohn’s Disease, inflammatory bowel disease and more.
A significant part of his work, especially in recent years, has focused on creating a delivery system for therapeutics that use synthetic nucleic acids to target and shut down specific, harmful genes and prevent viruses from spreading. These types of treatments, based on what are called siRNA, are exciting because they can be fine-tuned to hinder many different kinds of genes in the body by targeting the mRNA of a virus, which tell cells what to do.
The siRNA work builds on several previous papers that have come out of Peppas’ lab focused on developing hydrogels and nanoparticles for drug delivery.
A big chunk of Peppas’ work focuses on methods for oral delivery of treatments. However, different patients prefer different methods of treatment; for example; some people can’t swallow pills. So, it’s important for the field to recognize that and create diverse delivery vehicles.
Some people hate pills and love injections instead, and that tells you the patient factor is an extremely important factor,
said Peppas, who is a professor in the Department of Biomedical Engineering, McKetta Department of Chemical Engineering and also has appointments in the College of Pharmacy and Dell Medical School.
One of the main problems in the field of drug delivery is finding a way to not only protect a treatment as it is being injected but also as it is traveling to its target within the body. These dual demands require versatility in the delivery vehicle.
Adrianne Rosales, assistant professor of chemical engineering, is looking at materials that can morph and change their properties to handle these requirements. And the materials should be able to sense changing conditions in the body.
Stem cells have incredible potential for a wide variety of treatments, include bone regeneration and blood vessel growth. They are primarily delivered today in an aqueous solution, but that requires so many stem cells because they aren’t well protected along the way. Many of them never make it to their target in the body.
Rosales is investigating gels as a delivery vehicle that can both help them get into the body and serve as a scaffolding to protect them from the immune system’s natural defenses.
The ability to dynamically switch properties of the delivery vehicles is critical,
Rosales said. During injection you want one set of properties, but afterward, you might want another set of totally different properties.
Up Next: Low-cost and Universal Coronavirus Vaccines, and More
With several COVID-19 vaccines using UT Austin research receiving complete or emergency use approvals, the researchers are looking at the next step. Earlier this year, human trials began in several countries for a promising vaccine candidate that can be easily manufactured and stored in parts of the world that today rely on importing vaccines.
The vaccine, NDV-HXP-S, can be stored in a refrigerator and is made in chicken eggs, a method also used for flu vaccine manufacturing. These factors mean that affordable manufacturing capacity already exists in each of the countries involved with trials.
This vaccine uses a newer version of the spike protein created by UT Austin researchers, known as HexaPro, than the one present in the Moderna, Pfizer and other early COVID vaccines. HexaPro plays an important role in another major push at UT Austin: developing a catchall coronavirus vaccine that could treat everything from COVID-19 to MERS and SARS.
Researchers are developing a catchall coronavirus vaccine.
The key to creating a vaccine that can defeat all coronaviruses is to find antibodies that will bond with different types of spike proteins.
Spike is the first step in infection, so if we can block this first step we can block all the downstream effects of infection,
Maynard said.
Vaccine efforts, as well as other COVID treatments, involve wide-ranging collaborations between schools across UT Austin, as well as many outside partners. Beyond vaccines, another major project of interest is an effort that includes several faculty from the College of Pharmacy focused on delivery of COVID therapeutic antibodies by inhalation.
It’ll be interesting to see if the rapid mobilization of COVID vaccinations will have any long-term impact in the field. Will funding became more plentiful? Will it be easier to recruit faculty and students into vaccine development? Will regulatory agencies find a way to speed up approval timelines while continuing to ensure treatments are safe?
Rosales is somewhat new to the field, but she has noticed increased excitement among students lately. The speed with which the COVID vaccine came together seems to be motivating people who like the idea of seeing their research make a difference.
Everyone was floored by how quickly the vaccines came out,” she said. “That is very attractive to students, seeing the immediate impact their research can have, and therapeutics is an area where I’ve definitely seen a rise in student interest as a result.
And that increased interest is key, says Maynard. Getting more students and more faculty involved means that the team is more resilient in the case of retirements and changes within the research groups.
“We want to build it so that it becomes an enduring area of strength,” Maynard said, “that when you say ‘I want to do protein therapeutics’ you think about UT.”
by Nat Levy






